Phase Change
Storyboard 
One of the phase change processes is the evaporation process. In the liquid it absorbs energy by causing molecules of this to enter the state of gas that we call steam.
To model, we work with the Clausius Clapeyron equation that allows us to estimate the vapor pressure in equilibrium with the liquid.
ID:(316, 0)
Phase diagram of water
Image 
One of the most relevant phase diagrams for our planet is that of water. This diagram exhibits the three classical phases: solid, liquid, and gas, along with several phases featuring different crystalline structures of ice.
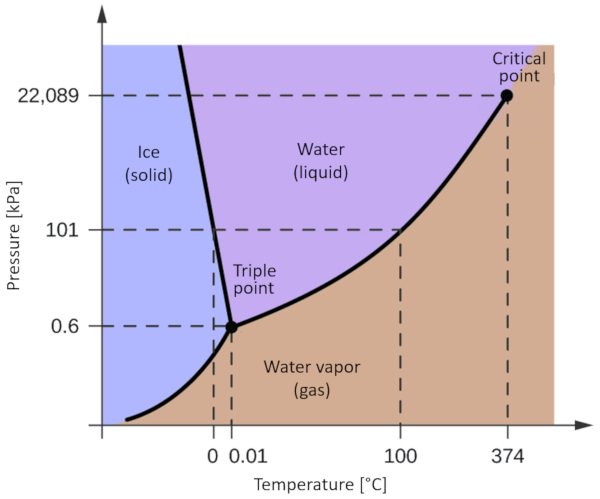
The significant distinction compared to other materials is that within a pressure range spanning from 611 Pa to 209.9 MPa, the solid phase occupies a greater volume than the liquid phase. This characteristic is reflected in the phase diagram as a negative slope along the boundary line separating the solid phase (hexagonal ice) and the liquid phase (water).
ID:(836, 0)
Clausius Clapeyron's law
Note 
If the differential of the Gibbs free energy ($dG$) is constant, this means that for the pressure Variation ($dp$) and the temperature variation ($dT$), the values of the entropy ($S$) and the volume ($V$) in phase 1
$dG = -S_1dT+V_1dp$
and the entropy ($S$) and the volume ($V$) in phase 2
$dG = -S_2dT+V_2dp$
result in
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{S_2-S_1}{V_2-V_1}$
The change in the entropy ($S$) between both phases corresponds to the latent Heat ($L$) divided by the absolute temperature ($T$):
$S_2 - S_1 =\displaystyle\frac{ L }{ T }$
Then, with the definition of the volume variation in phase change ($\Delta V$)
$\Delta V \equiv V_2 - V_1$
we obtain the Clausius-Clapeyron equation [1,2,3]
| $\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T }$ |
![]() [1] "Über die Art der Bewegung, welche wir Wärme nennen" (On the Kind of Motion We Call Heat), Rudolf Clausius, Annalen der Physik und Chemie, 155(6), 368-397. (1850)
[1] "Über die Art der Bewegung, welche wir Wärme nennen" (On the Kind of Motion We Call Heat), Rudolf Clausius, Annalen der Physik und Chemie, 155(6), 368-397. (1850)
![]() [2] "Ueber eine veränderte Form des zweiten Hauptsatzes der mechanischen Wärmetheorie" (On a Modified Form of the Second Law of the Mechanical Theory of Heat), Rudolf Clausius, Annalen der Physik, 176(3), 353-400. (1857)
[2] "Ueber eine veränderte Form des zweiten Hauptsatzes der mechanischen Wärmetheorie" (On a Modified Form of the Second Law of the Mechanical Theory of Heat), Rudolf Clausius, Annalen der Physik, 176(3), 353-400. (1857)
![]() [3] "Mémoire sur la puissance motrice de la chaleur" (Memoir on the Motive Power of Heat), Benoît Paul Émile Clapeyron, Journal de l'École Royale Polytechnique, 14, 153-190. (1834)
[3] "Mémoire sur la puissance motrice de la chaleur" (Memoir on the Motive Power of Heat), Benoît Paul Émile Clapeyron, Journal de l'École Royale Polytechnique, 14, 153-190. (1834)
ID:(15765, 0)
Molar Clausius Clapeyron law
Quote 
With the Clausius-Clapeyron law, which depends on the pressure Variation ($dp$), the temperature variation ($dT$), the latent Heat ($L$), the volume variation in phase change ($\Delta V$), and the absolute temperature ($T$), expressed as:
| $\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T }$ |
and the definition of the molar Latent Heat ($l_m$), where the latent Heat ($L$) is related to the molar Mass ($M_m$) as follows:
| $ l_m \equiv\displaystyle\frac{ L }{ M_m }$ |
and the variation of molar volume during phase change ($\Delta v_m$), where the volume variation in phase change ($\Delta V$) is related to the molar Mass ($M_m$) as follows:
| $\Delta v_m =\displaystyle\frac{ \Delta V }{ M_m }$ |
we obtain:
| $\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ l_m }{ \Delta v_m T }$ |
ID:(15766, 0)
Rudolf Clausius
Equation 
Rudolf Clausius (1822-1888) was a German physicist and mathematician who made significant contributions to the field of thermodynamics. He is best known for formulating the second law of thermodynamics and for introducing the concept of entropy as a fundamental quantity in the study of energy transfer and transformation in physical systems.

ID:(1885, 0)
Phase Changes
Variable 
One of the phenomena that also includes thermodynamics is the study of state changes. There are three basic states, gas, liquid and solid.
In the case of gas, the particles move almost freely and interact only with sporadic shocks.
In the case of a liquid the interaction is permanent. Despite this, the particles can be displaced without permanent unions towards the neighbors.
In the case of the solid, the interactions are such that the particles can no longer be displaced and only oscillate around a point of equilibrium defined by the neighboring particles.
The study of the changes of state tries to explain why the material passes in one form to the other and vice versa. In the case of medicine, these changes are relevant in processes where, for example, substances evaporate or dissolve. This both for the removal of materials and for temperature reduction such as perspiration.
ID:(147, 0)
Special Case of Water
Audio 
Water is a special case since its phase diagram presents a key difference with respect to the vast majority of materials: the line that separates the solid and liquid phases has a negative slope.
This is due to the fact that the water changes from solid to liquid reduces its volume and with it the variation in volume $ \ Delta V $ is negative and the slope in the Clausius Clapeyron equation ends up being negative.
This property of water leads to the fact that if the temperature is lowered when it is in a liquid state $(dT,negative)$ upon reaching the phase change the pressure increases $ (dp) $ while in a normal liquid it is reduced . This is what leads to that in winter for example
freezing the water accumulated in the cracks and cracks of rocks in the mountains generates pressure which leads to its rupture and ultimately to the erosion of our geography.
When the surface of a lake freezes, the lake's water encapsulates, preventing it from expanding to form ice. This prevents lakes from freezing to the bottom while preserving life inside.
ID:(146, 0)
Phase Change
Storyboard 
One of the phase change processes is the evaporation process. In the liquid it absorbs energy by causing molecules of this to enter the state of gas that we call steam. To model, we work with the Clausius Clapeyron equation that allows us to estimate the vapor pressure in equilibrium with the liquid.
Variables
Calculations
Calculations
Equations
With the Clausius-Clapeyron law, which depends on the pressure Variation ($dp$), the temperature variation ($dT$), the latent Heat ($L$), the volume variation in phase change ($\Delta V$), and the absolute temperature ($T$), expressed as:
and the definition of the molar Latent Heat ($l_m$), where the latent Heat ($L$) is related to the molar Mass ($M_m$) as follows:
and the variation of molar volume during phase change ($\Delta v_m$), where the volume variation in phase change ($\Delta V$) is related to the molar Mass ($M_m$) as follows:
we obtain:
If the differential of the Gibbs free energy ($dG$) is constant, it means that for the pressure Variation ($dp$) and the temperature variation ($dT$), the values of the entropy ($S$) and the volume ($V$) in phase 1
$dG = -S_1dT+V_1dp$
and the entropy ($S$) and the volume ($V$) in phase 2
$dG = -S_2dT+V_2dp$
yield
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{S_2-S_1}{V_2-V_1}$
The change in the entropy ($S$) between both phases corresponds to the latent Heat ($L$) divided by the absolute temperature ($T$):
$S_2 - S_1 =\displaystyle\frac{ L }{ T }$
So, with the definition of the volume variation in phase change ($\Delta V$)
$\Delta V \equiv V_2 - V_1$
we obtain
Examples
One of the most relevant phase diagrams for our planet is that of water. This diagram exhibits the three classical phases: solid, liquid, and gas, along with several phases featuring different crystalline structures of ice.
The significant distinction compared to other materials is that within a pressure range spanning from 611 Pa to 209.9 MPa, the solid phase occupies a greater volume than the liquid phase. This characteristic is reflected in the phase diagram as a negative slope along the boundary line separating the solid phase (hexagonal ice) and the liquid phase (water).
If the differential of the Gibbs free energy ($dG$) is constant, this means that for the pressure Variation ($dp$) and the temperature variation ($dT$), the values of the entropy ($S$) and the volume ($V$) in phase 1
$dG = -S_1dT+V_1dp$
and the entropy ($S$) and the volume ($V$) in phase 2
$dG = -S_2dT+V_2dp$
result in
$\displaystyle\frac{dp}{dT}=\displaystyle\frac{S_2-S_1}{V_2-V_1}$
The change in the entropy ($S$) between both phases corresponds to the latent Heat ($L$) divided by the absolute temperature ($T$):
$S_2 - S_1 =\displaystyle\frac{ L }{ T }$
Then, with the definition of the volume variation in phase change ($\Delta V$)
$\Delta V \equiv V_2 - V_1$
we obtain the Clausius-Clapeyron equation [1,2,3]
![]() [1] " ber die Art der Bewegung, welche wir W rme nennen" (On the Kind of Motion We Call Heat), Rudolf Clausius, Annalen der Physik und Chemie, 155(6), 368-397. (1850)
[1] " ber die Art der Bewegung, welche wir W rme nennen" (On the Kind of Motion We Call Heat), Rudolf Clausius, Annalen der Physik und Chemie, 155(6), 368-397. (1850)
![]() [2] "Ueber eine ver nderte Form des zweiten Hauptsatzes der mechanischen W rmetheorie" (On a Modified Form of the Second Law of the Mechanical Theory of Heat), Rudolf Clausius, Annalen der Physik, 176(3), 353-400. (1857)
[2] "Ueber eine ver nderte Form des zweiten Hauptsatzes der mechanischen W rmetheorie" (On a Modified Form of the Second Law of the Mechanical Theory of Heat), Rudolf Clausius, Annalen der Physik, 176(3), 353-400. (1857)
![]() [3] "M moire sur la puissance motrice de la chaleur" (Memoir on the Motive Power of Heat), Beno t Paul mile Clapeyron, Journal de l' cole Royale Polytechnique, 14, 153-190. (1834)
[3] "M moire sur la puissance motrice de la chaleur" (Memoir on the Motive Power of Heat), Beno t Paul mile Clapeyron, Journal de l' cole Royale Polytechnique, 14, 153-190. (1834)
With the Clausius-Clapeyron law, which depends on the pressure Variation ($dp$), the temperature variation ($dT$), the latent Heat ($L$), the volume variation in phase change ($\Delta V$), and the absolute temperature ($T$), expressed as:
and the definition of the molar Latent Heat ($l_m$), where the latent Heat ($L$) is related to the molar Mass ($M_m$) as follows:
and the variation of molar volume during phase change ($\Delta v_m$), where the volume variation in phase change ($\Delta V$) is related to the molar Mass ($M_m$) as follows:
we obtain:
One of the phenomena that also includes thermodynamics is the study of state changes. There are three basic states, gas, liquid and solid.
In the case of gas, the particles move almost freely and interact only with sporadic shocks.
In the case of a liquid the interaction is permanent. Despite this, the particles can be displaced without permanent unions towards the neighbors.
In the case of the solid, the interactions are such that the particles can no longer be displaced and only oscillate around a point of equilibrium defined by the neighboring particles.
The study of the changes of state tries to explain why the material passes in one form to the other and vice versa. In the case of medicine, these changes are relevant in processes where, for example, substances evaporate or dissolve. This both for the removal of materials and for temperature reduction such as perspiration.
Water is a special case since its phase diagram presents a key difference with respect to the vast majority of materials: the line that separates the solid and liquid phases has a negative slope.
This is due to the fact that the water changes from solid to liquid reduces its volume and with it the variation in volume $ \ Delta V $ is negative and the slope in the Clausius Clapeyron equation ends up being negative.
This property of water leads to the fact that if the temperature is lowered when it is in a liquid state $(dT,negative)$ upon reaching the phase change the pressure increases $ (dp) $ while in a normal liquid it is reduced . This is what leads to that in winter for example
freezing the water accumulated in the cracks and cracks of rocks in the mountains generates pressure which leads to its rupture and ultimately to the erosion of our geography.
When the surface of a lake freezes, the lake's water encapsulates, preventing it from expanding to form ice. This prevents lakes from freezing to the bottom while preserving life inside.
Rudolf Clausius (1822-1888) was a German physicist and mathematician who made significant contributions to the field of thermodynamics. He is best known for formulating the second law of thermodynamics and for introducing the concept of entropy as a fundamental quantity in the study of energy transfer and transformation in physical systems.
The evaporated Mass ($\Delta m$) is defined using the latent Heat ($L$) and the phase Change Heat ($\Delta Q$) as follows:
The Clausius-Clapeyron law establishes a relationship between the pressure Variation ($dp$) and the temperature variation ($dT$) with the latent Heat ($L$), the absolute temperature ($T$) and the volume variation in phase change ($\Delta V$) as follows:
In many cases the latent molar heat is not available but the latent heat that is expressed, for example, in Joules per kilogram (J/Kg). Since the vapor pressure equation works with the latent molar heat we must convert the latent heat into latent molar heat. Since the latter is per mole, it is enough to divide the latent heat
In the case of water, the latent heat of evaporation is of the order of
The change in volume between the material in two different states can be expressed in moles
to obtain a characteristic indicator of the material.
The Clausius-Clapeyron equation establishes a relationship between the pressure Variation ($dp$) and the temperature variation ($dT$) with the absolute temperature ($T$), the molar Latent Heat ($l_m$) and the variation of molar volume during phase change ($\Delta v_m$) as follows:
ID:(316, 0)
