Refrigerators
Storyboard 
Refrigeration is an inverse process of the internal combustion machine. In this case, work is supplied by which heat is extracted from a thermodynamic system.
ID:(1489, 0)
Elements of a refrigerator
Definition 
If you observe a refrigerator, you can relatively easily identify the different parts that compose it. These are:
• Evaporator (which absorbs heat). This component operates by evaporating the refrigerant liquid to absorb heat from inside the refrigerator.
• Compressor. The compressor transfers the vapor generated by the evaporator, compresses it, and pushes it towards the condenser.
• Condenser (which emits heat). In the condenser, the vapor condenses, releasing energy that is radiated into the environment through the grille usually located at the back of the refrigerator.
• Expansion valve, which allows the liquid to return to the evaporator.

ID:(11167, 0)
Heat pump application: the refrigerator
Image 
If you observe a refrigerator, you can relatively easily identify the different parts that compose it. These are:
• Evaporator (which absorbs heat). This component operates by evaporating the refrigerant liquid to absorb heat from inside the refrigerator.
• Compressor. The compressor transfers the vapor generated by the evaporator, compresses it, and pushes it towards the condenser.
• Condenser (which emits heat). In the condenser, the vapor condenses, releasing energy that is radiated into the environment through the grille usually located at the back of the refrigerator.
• Expansion valve, which allows the liquid to return to the evaporator.
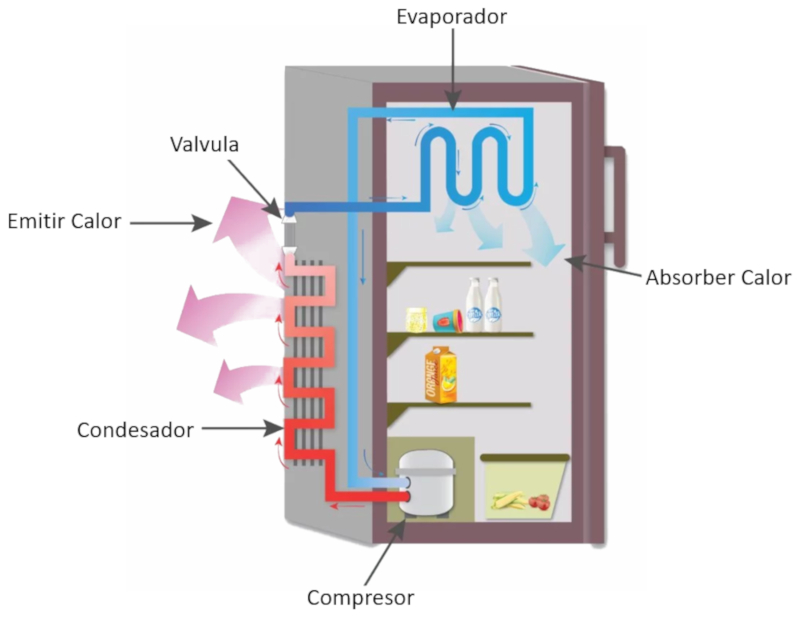
ID:(11166, 0)
Carnot cycle for refrigeration
Exercise 
If the Carnot process is reversed, it can be used to transfer heat using work, which is known as a heat pump. In this case, the diagram is as follows:
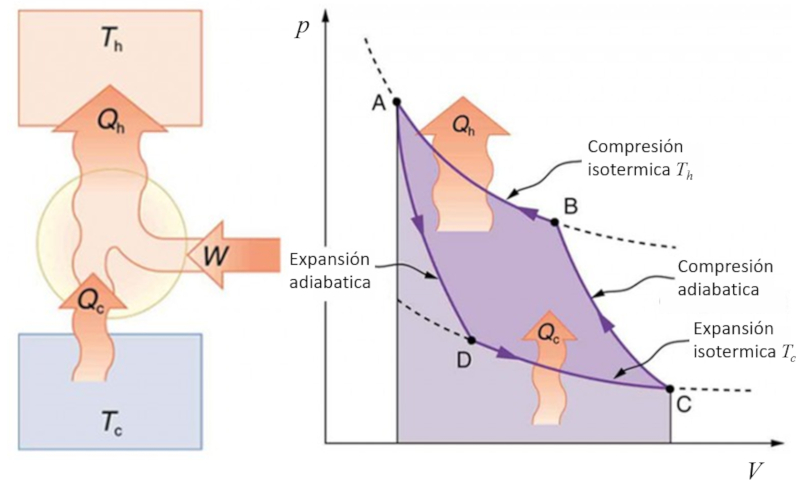
ID:(11143, 0)
Concept of a heat pump
Equation 
A heat pump is a way to implement the reverse Carnot process, which involves using work to transfer heat from a colder body to a hotter one.
A heat pump includes two key components:
• An evaporator (which absorbs heat)
• A compressor (which releases heat)
It also has a circuit to move the vapor and liquid:
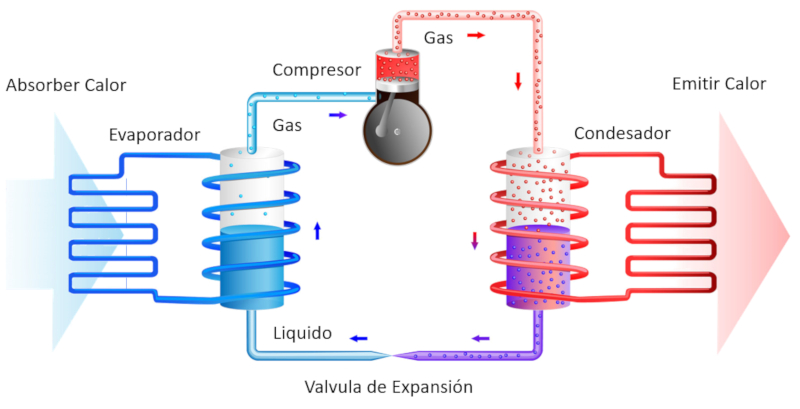
ID:(11165, 0)
Temperature and entropy diagram in case of refrigeration
Script 
The temperature-entropy diagram contains three regions that represent different states. These are:
• Liquid
• Vapor
• Liquid-vapor mixture
The process itself involves the following stages:
1 to 2: Application of work
2 to 3: Heat rejection in the condenser
3 to 4: Expansion in the expansion valve
4 to 1: Heat absorption in the evaporator

ID:(11168, 0)
Refrigerators
Description 
Refrigeration is an inverse process of the internal combustion machine. In this case, work is supplied by which heat is extracted from a thermodynamic system.
Variables
Calculations
Calculations
Equations
Examples
If you observe a refrigerator, you can relatively easily identify the different parts that compose it. These are:
• Evaporator (which absorbs heat). This component operates by evaporating the refrigerant liquid to absorb heat from inside the refrigerator.
• Compressor. The compressor transfers the vapor generated by the evaporator, compresses it, and pushes it towards the condenser.
• Condenser (which emits heat). In the condenser, the vapor condenses, releasing energy that is radiated into the environment through the grille usually located at the back of the refrigerator.
• Expansion valve, which allows the liquid to return to the evaporator.

(ID 11167)
If you observe a refrigerator, you can relatively easily identify the different parts that compose it. These are:
• Evaporator (which absorbs heat). This component operates by evaporating the refrigerant liquid to absorb heat from inside the refrigerator.
• Compressor. The compressor transfers the vapor generated by the evaporator, compresses it, and pushes it towards the condenser.
• Condenser (which emits heat). In the condenser, the vapor condenses, releasing energy that is radiated into the environment through the grille usually located at the back of the refrigerator.
• Expansion valve, which allows the liquid to return to the evaporator.

(ID 11166)
The heat required to restore the system is calculated directly by integrating the entropy from
| $ W =\displaystyle\oint_C T dS $ |
from $S_H$ to $S_L$.
| $ Q_C = T_C ( S_H - S_C )$ |
(ID 10263)
(ID 15285)
(ID 15344)
If the Carnot process is reversed, it can be used to transfer heat using work, which is known as a heat pump. In this case, the diagram is as follows:

(ID 11143)
A heat pump is a way to implement the reverse Carnot process, which involves using work to transfer heat from a colder body to a hotter one.
A heat pump includes two key components:
• An evaporator (which absorbs heat)
• A compressor (which releases heat)
It also has a circuit to move the vapor and liquid:

(ID 11165)
The temperature-entropy diagram contains three regions that represent different states. These are:
• Liquid
• Vapor
• Liquid-vapor mixture
The process itself involves the following stages:
1 to 2: Application of work
2 to 3: Heat rejection in the condenser
3 to 4: Expansion in the expansion valve
4 to 1: Heat absorption in the evaporator

(ID 11168)
ID:(1489, 0)
