Collisions between Particles
Storyboard 
The most basic form of interaction are collisions between particles. These will depend on the size of the particles and their concentration.
ID:(1496, 0)
Collisions between Particles
Storyboard 
The most basic form of interaction are collisions between particles. These will depend on the size of the particles and their concentration.
Variables
Calculations
Calculations
Equations
Given the particle concentration ($c_n$) with the number of particles ($N$) and the volume ($V$), we have:
With the particle mass ($m$) and the mass ($M$),
As the density ($\rho$) is
we obtain
$c_n=\displaystyle\frac{N}{V}=\displaystyle\frac{M}{mV}=\displaystyle\frac{\rho}{m}$
Therefore,
Examples
If we divide the density ($\rho$) by the particle mass ($m$), we will obtain the particle concentration ($c_n$):
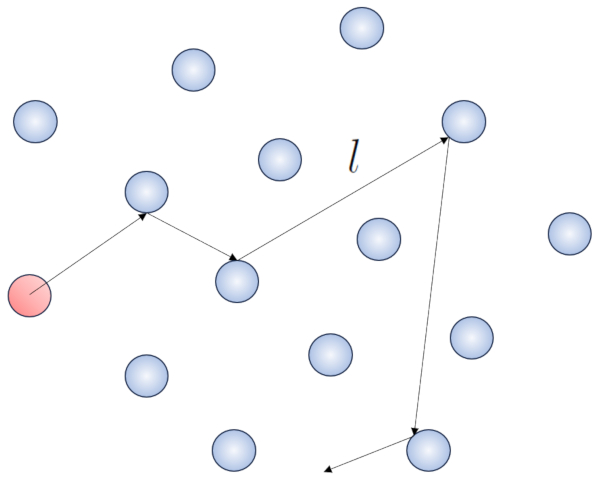
When a particle of a gas moves, it interacts with other particles. The simplest form of this interaction is through elastic collisions, meaning that the particle collides without losing energy, changing its direction to impact another particle.
Within this process, it makes sense to define the free path ($\bar{l}$), whose value will depend on ERROR:5548.1.
When a molecule moves periodically through the volume containing the gas, it will eventually encounter another molecule and they may collide. The distance it travels between two consecutive collisions is called the 'mean free path'.
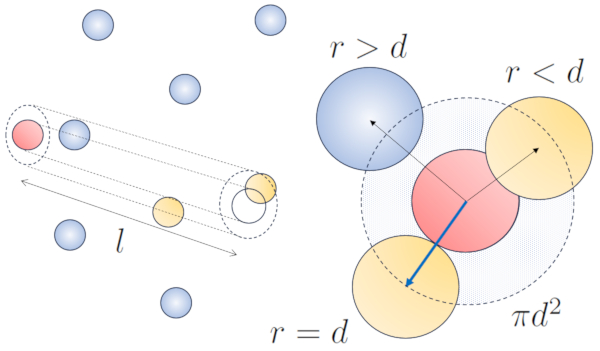
When a particle with a given radius moves through space, it effectively occupies the space of a cylinder with the same radius. For one particle to collide with another, the second particle must have some of its volume within this cylinder. In the most extreme case, the second particle is located at a distance of two radii from the first one, so that the edge of the cylinder touches a point on the sphere closest to the cylinder's axis. The center of this sphere is at a distance equal to one radius from the surface of the cylinder:
Therefore, the distance between the cylinder's axis and the center of any particle is two radii, or in other words, a diameter. In essence, one can imagine that the volume literally occupied by the particle traveling through space is a cylinder with a length equal to the free path and a radius equal to the particle's diameter.
Since the diameter of the particle $d$ is twice the radius $a$
$d=2a$
and the particle concentration $c_N$ can be expressed in terms of molar concentration $c_n$ as
$c_N=N_Ac_n$
where $N_A$ is Avogadro's number, the equation for mean free path
$l=\displaystyle\frac{1}{4a^2\pi c_N}$
can also be written as:
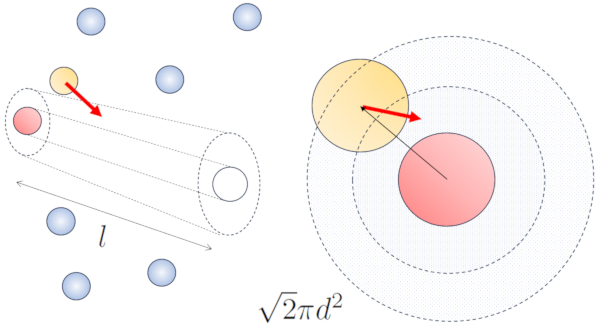
When neighboring particles are in motion, there is a higher probability of collision due to the fact that they cover a greater distance in the same amount of time. The velocity components, $v_x$, $v_y$, and $v_z$, fluctuate around mean values $\sqrt{\langle v_x^2\rangle}$, $\sqrt{\langle v_y^2\rangle}$, and $\sqrt{\langle v_z^2\rangle}$. Assuming the system is isotropic, the average of each component will be equal to $\displaystyle\frac{1}{3}\sqrt{\langle v^2\rangle}$. Therefore, along the axis along which the particle is moving, it will travel a distance
$\sqrt{\langle v_z^2\rangle}dt=\displaystyle\frac{1}{3}\sqrt{\langle v^2\rangle}dt$
At the same time, particles moving perpendicular will have covered a distance:
$\sqrt{\langle v_x^2\rangle+\langle v_y^2\rangle}dt=\displaystyle\frac{\sqrt{2}}{3}\sqrt{\langle v^2\rangle}dt$
Hence, the collision probability increases by a factor of $\sqrt{2}$ compared to the case where the particles are not in motion:
The mean free path can be estimated in terms of the diameter of an imaginary cylinder surrounding a particle, on average having one collision with another particle.
The radius of the cylinder corresponds to the maximum distance two particles must have to collide, which is equal to twice the radius of the particle, i.e., the particle diameter ($d$). Since only one collision occurs within this cylinder, the number of particles contained within it must be equal to one. This means that:
$l d^2\pi c_n= 1$
with the particle concentration ($c_n$), and solving for the free path ($\bar{l}$), we obtain:
This represents the mean free path.
For the case without movement, the probability is the free path ($\bar{l}$), whereas with movement, the probabilities are the particle diameter ($d$) and the particle concentration ($c_n$), respectively.
In the case of movement, the probability increases by a factor of $\sqrt{2}$, which means the free path is
If
$l\pi d^2c_n=1$
or
The molar concentration ($c_m$) corresponds to ERROR:9339,0 divided by the volume ($V$) of a gas and is calculated as follows:
To convert the molar concentration ($c_m$) to the particle concentration ($c_n$), simply multiply the former by the avogadro's number ($N_A$) as follows:
ID:(1496, 0)