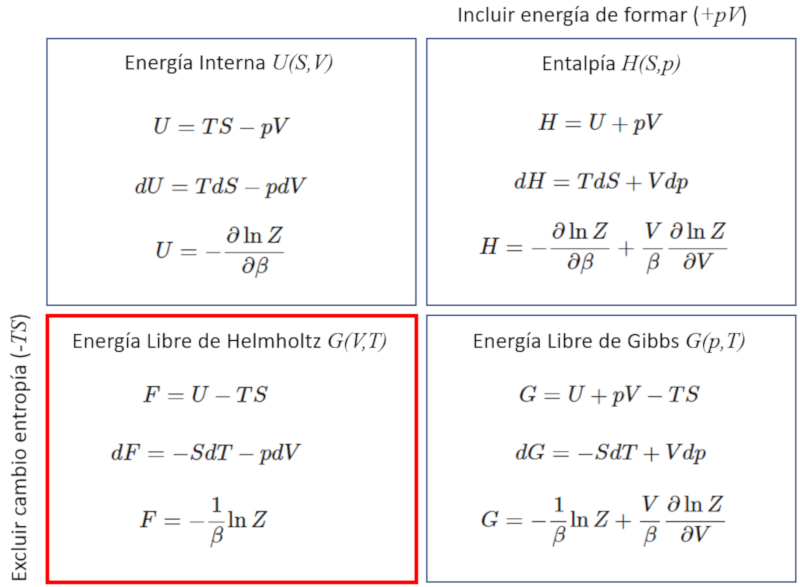
Energía Líbre
Storyboard 
Se obtienen mediante la función partición las distintas funciones y relaciones termodinámicas.
ID:(442, 0)
Helmholtz free energy with partition function
Definition 
As the derivative with respect to the volume of the free energy of Helmholtz at constant temperature is:
ID:(11725, 0)
Energía Líbre
Storyboard 
Se obtienen mediante la función partición las distintas funciones y relaciones termodinámicas.
Variables
Calculations
Calculations
Equations
The helmholtz free fnergy ($F$) is defined using the internal energy ($U$), the absolute temperature ($T$), and the entropy ($S$) as:
When we differentiate this equation, we obtain with the differential Helmholtz Free Energy ($dF$), the variation of the internal energy ($dU$), the entropy variation ($dS$), and the temperature variation ($dT$):
$dF = dU - TdS - SdT$
With the differential of internal energy and the variables the pressure ($p$) and the volume Variation ($\Delta V$),
we finally obtain:
The differential Helmholtz Free Energy ($dF$) is a function of the variations of the absolute temperature ($T$) and the volume ($V$), as well as the slopes the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) and the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$), expressed as:
Comparing this with the equation for the differential Helmholtz Free Energy ($dF$):
and with the first law of thermodynamics, it follows that the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) is equal to negative the entropy ($S$):
The differential Helmholtz Free Energy ($dF$) is a function of the variations of the absolute temperature ($T$) and the volume ($V$), as well as the slopes the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) and the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$), which is expressed as:
Comparing this with the equation for the differential Helmholtz Free Energy ($dF$):
and with the first law of thermodynamics, it follows that the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$) is equal to negative the pressure ($p$):
Since the differential Helmholtz Free Energy ($dF$) is an exact differential, we should note that the helmholtz free fnergy ($F$) with respect to the absolute temperature ($T$) and the volume ($V$) must be independent of the order in which the function is derived:
$D(DF_{T,V})_{V,T}=D(DF{V,T})_{T,V}$
Using the relationship between the slope the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) and the entropy ($S$)
and the relationship between the slope the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$) and the pressure ($p$)
we can conclude that:
Given that the helmholtz free fnergy ($F$) depends on the absolute temperature ($T$) and the volume ($V$), the differential Helmholtz Free Energy ($dF$) can be calculated using:
$dF = \left(\displaystyle\frac{\partial F}{\partial T}\right)_V dT + \left(\displaystyle\frac{\partial F}{\partial V}\right)_T dV$
To simplify this expression, we introduce the notation for the derivative of the helmholtz free fnergy ($F$) with respect to the absolute temperature ($T$) while keeping the volume ($V$) constant as:
$DF_{T,V} \equiv \left(\displaystyle\frac{\partial F}{\partial T}\right)_V$
and for the derivative of the helmholtz free fnergy ($F$) with respect to the volume ($V$) while keeping the absolute temperature ($T$) constant as:
$DF_{V,T} \equiv \left(\displaystyle\frac{\partial F}{\partial V}\right)_T$
thus we can write:
Examples
As the derivative with respect to the volume of the free energy of Helmholtz at constant temperature is:
The dependency of the differential Helmholtz Free Energy ($dF$) on the entropy ($S$) and the temperature variation ($dT$), in addition to the pressure ($p$) and the volume Variation ($\Delta V$), is given by:
The differential Helmholtz Free Energy ($dF$) is a function of the variations of the absolute temperature ($T$) and the volume ($V$), as well as the slopes the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) and the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$), which is expressed as:
La derivada de la energ a interna en el volumen a entropia constante es
La derivada de la energ a interna en el volumen a entropia constante es
Comparing this with the first law of thermodynamics, it turns out that the partial derivative of the Helmholtz free energy with respect to temperature at constant volume ($DF_{T,V}$) is equal to minus the entropy ($S$):
Comparing this with the first law of thermodynamics, it turns out that the partial derivative of the Helmholtz free energy with respect to volume at constant temperature ($DF_{V,T}$) is equal to minus the pressure ($p$):
Como la derivada respecto del volumen de la energ a libre de Helmholtz a temperatura constante es con
y la presi n es con
se tiene que la energ a libre de Helmholtz es con
With the entropy ($S$), the volume ($V$), the absolute temperature ($T$) and the pressure ($p$) we obtain one of the so-called Maxwell relations:
La derivada de la entrop a en el volumen a temperatura constante es
La derivada de la presi n en la temperatura a volumen constante es
ID:(442, 0)
