Energía Libre de Gibbs
Storyboard 
Se obtienen mediante la función partición las distintas funciones y relaciones termodinámicas.
ID:(443, 0)
Gibbs free energy with partition function
Image 
To calculate the Gibbs function of the partition function, it is enough to see how the enthalpy and the entropy of it are constructed. How do you have to
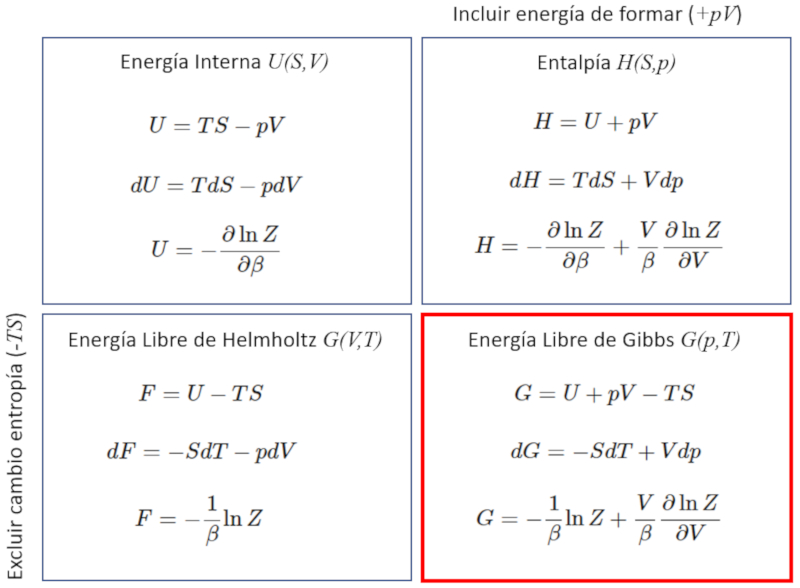
ID:(11726, 0)
Energía Libre de Gibbs
Description 
Se obtienen mediante la función partición las distintas funciones y relaciones termodinámicas.
Variables
Calculations
Calculations
Equations
The gibbs free energy ($G$) as a function of the enthalpy ($H$), the entropy ($S$), and the absolute temperature ($T$) is expressed as:
| $ G = H - T S $ |
The value of the differential of the Gibbs free energy ($dG$) is determined using the differential enthalpy ($dH$), the temperature variation ($dT$), and the entropy variation ($dS$) through the equation:
$dG=dH-SdT-TdS$
Since the differential enthalpy ($dH$) is related to the volume ($V$) and the pressure Variation ($dp$) as follows:
| $ dH = T dS + V dp $ |
It follows that the differential enthalpy ($dH$), the entropy variation ($dS$), and the pressure Variation ($dp$) are interconnected in the following manner:
| $ dG =- S dT + V dp $ |
(ID 3541)
(ID 3542)
The differential of the Gibbs free energy ($dG$) is a function of the variations of the absolute temperature ($T$) and the pressure ($p$), as well as the slopes the partial derivative of the Gibbs free energy with respect to temperature at constant pressure ($DG_{T,p}$) and the partial derivative of the Gibbs free energy with respect to pressure at constant temperature ($DG_{p,T}$), expressed as:
| $ dG = DG_{T,p} dT + DG_{p,T} dp $ |
Comparing this with the equation for the variation of Gibbs Free Energy ($dG$):
| $ dG =- S dT + V dp $ |
and with the first law of thermodynamics, it follows that the partial derivative of the Gibbs free energy with respect to temperature at constant pressure ($DG_{T,p}$) is equal to negative the entropy ($S$):
| $ DG_{T,p} =- S $ |
(ID 3552)
The differential of the Gibbs free energy ($dG$) is a function of the variations of the absolute temperature ($T$) and the pressure ($p$), as well as the slopes the partial derivative of the Gibbs free energy with respect to temperature at constant pressure ($DG_{T,p}$) and the partial derivative of the Gibbs free energy with respect to pressure at constant temperature ($DG_{p,T}$), expressed as:
| $ dG = DG_{T,p} dT + DG_{p,T} dp $ |
Comparing this with the equation for the variation of Gibbs Free Energy ($dG$):
| $ dG =- S dT + V dp $ |
and with the first law of thermodynamics, it follows that the partial derivative of the Gibbs free energy with respect to pressure at constant temperature ($DG_{p,T}$) is equal to the volume ($V$):
| $ DG_{p,T} = V $ |
(ID 3553)
Since the differential of the Gibbs free energy ($dG$) is an exact differential, it implies that the gibbs free energy ($G$) with respect to the absolute temperature ($T$) and the pressure ($p$) must be independent of the order in which the function is derived:
$D(DG_{T,p}){p,T}=D(DG{p,T})_{T,p}$
Using the relationship for the slope the partial derivative of the Gibbs free energy with respect to pressure at constant temperature ($DG_{p,T}$) with respect to the volume ($V$)
| $ DG_{p,T} = V $ |
and the relationship for the slope the partial derivative of the Gibbs free energy with respect to temperature at constant pressure ($DG_{T,p}$) with respect to the entropy ($S$)
| $ DG_{T,p} =- S $ |
we can conclude that:
| $ DS_{p,T} = -DV_{T,p} $ |
(ID 3557)
Given that the gibbs free energy ($G$) depends on the absolute temperature ($T$) and the pressure ($p$), the variation of Gibbs Free Energy ($dG$) can be calculated using:
$dG = \left(\displaystyle\frac{\partial G}{\partial T}\right)_p dT + \left(\displaystyle\frac{\partial G}{\partial p}\right)_T dp$
To simplify this expression, we introduce the notation for the derivative of the gibbs free energy ($G$) with respect to the absolute temperature ($T$) while keeping the pressure ($p$) constant as:
$DG_{T,p} \equiv \left(\displaystyle\frac{\partial G}{\partial T}\right)_p$
and for the derivative of the gibbs free energy ($G$) with respect to the pressure ($p$) while keeping the absolute temperature ($T$) constant as:
$DG_{p,T} \equiv \left(\displaystyle\frac{\partial G}{\partial p}\right)_T$
thus we can write:
| $ dG = DG_{T,p} dT + DG_{p,T} dp $ |
(ID 8188)
Examples
To calculate the Gibbs function of the partition function, it is enough to see how the enthalpy and the entropy of it are constructed. How do you have to

(ID 11726)
ID:(443, 0)
