Water Vapor on the Ground
Storyboard 
Normally the pressure of the real water vapor is lower than the maximum it can withstand before starting to condense it. Therefore, the concept of relative humidity is introduced.
ID:(377, 0)
Gas phase, water vapor
Concept 
The gaseous phase, which in our case corresponds to water vapor, is the phase in which atoms can move relatively freely.

In this phase, there is only minimal interaction that can affect the behavior of atoms without significantly confining them.
ID:(15142, 0)
Liquid phase, water
Concept 
The liquid phase, which in our case corresponds to water, is the phase in which atoms can move relatively freely while maintaining their unity and adapting to the shape that contains them.

In this phase, no specific structure is observed.
ID:(15140, 0)
Solid phase, ice
Concept 
The solid phase, which in our case corresponds to ice, is the phase in which atoms cannot move relatively and can only oscillate around their equilibrium point.

In this phase, one can observe a structure that is often crystalline and, therefore, regular.
ID:(15141, 0)
Phase diagram of water
Concept 
One of the most relevant phase diagrams for our planet is that of water. This diagram exhibits the three classical phases: solid, liquid, and gas, along with several phases featuring different crystalline structures of ice.
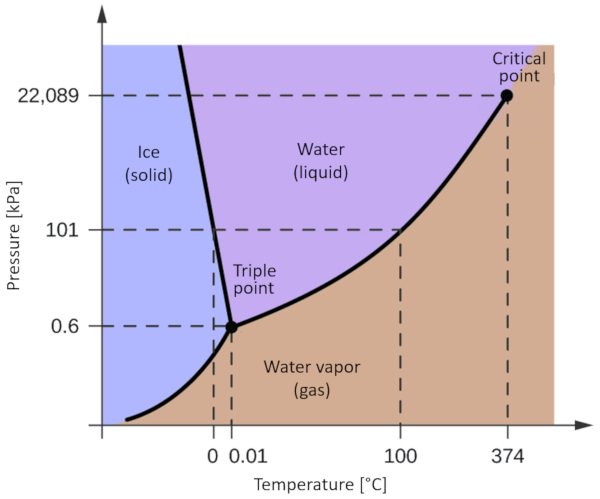
The significant distinction compared to other materials is that within a pressure range spanning from 611 Pa to 209.9 MPa, the solid phase occupies a greater volume than the liquid phase. This characteristic is reflected in the phase diagram as a negative slope along the boundary line separating the solid phase (hexagonal ice) and the liquid phase (water).
ID:(836, 0)
Evaporation heat measurement
Image 
The measurement of the heat of vaporization is carried out by heating a sample, causing it to evaporate, while simultaneously measuring the heat delivered to the sample. Then, the vapor is cooled and condensed back, and the mass that originally evaporated is measured.

In this way, we can estimate the energy required to evaporate a given mass, which corresponds to latent Heat (L) measured in joules per kilogram (J/kg) or joules per mole (J/mol).
ID:(1662, 0)
Water Vapor
Concept 
The gaseous phase of water corresponds to what is known as water vapor. It is created as water molecules acquire enough kinetic energy to escape from the liquid phase and begin to move through the space above the liquid. Periodically, the molecules in the gaseous state collide with the liquid surface again and are captured, returning to the liquid state.

As the number of molecules in the gaseous state increases, so does the number that returns to the liquid. This process continues until an equilibrium is reached between the molecules leaving the liquid and those being reabsorbed. In this situation, it is said that the space above the liquid is saturated.
ID:(1010, 0)
Model
Top 
Parameters
Variables
Calculations
Calculations
Calculations
Equations
dG =- S dT + V dp
dG =- S * dT + V * dp
dH = T dS + V dp
dH = T * dS + V * dp
\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T }
dp / dT = L /( DV * T )
\displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ l_m }{ \Delta v_m T }
dp / dT = l_m /( Dv_m * T )
\delta Q = T dS
dQ = T * dS
dU = \delta Q - \delta W
dU = dQ - dW
dU = T dS - p dV
dU = T * dS - p * dV
\delta W = p dV
dW = p * dV
H = U + p V
H = U + p * V
l_m \equiv\displaystyle\frac{ L }{ M_m }
l_m = L / M_m
p = c_m R T
p = c_m * R * T
p_s = p_{ref} e^{- l_m / R T }
p_s = p_ref *exp(- l_m / R * T )
RH =\displaystyle\frac{ c_v }{ c_s }
RH = c_v / c_s
RH =\displaystyle\frac{ p_v }{ p_s }
RH = p_v / p_s
ID:(15231, 0)
First law of thermodynamics
Equation 
The internal energy differential (dU) is always equal to the amount of the differential inexact Heat (\delta Q) supplied to the system (positive) minus the amount of the differential inexact labour (\delta W) performed by the system (negative):
ID:(9632, 0)
Work depending on the volume
Equation 
In analogy to the definition of work dW in mechanics:
| \delta W = F dx |
which is defined in terms of force F and displacement dx, in thermodynamics, we work with the expression of work in terms of pressure p and volume change dV:
ID:(9634, 0)
Second law of thermodynamics
Equation 
The differential inexact Heat (\delta Q) is equal to the absolute temperature (T) times the entropy variation (dS):
ID:(9639, 0)
Internal Energy: differential ratio
Equation 
The dependency of the internal energy differential (dU) on the pressure (p) and the volume Variation (dV), in addition to the absolute temperature (T) and the entropy variation (dS), is given by:
As the internal energy differential (dU) depends on the differential inexact Heat (\delta Q), the pressure (p), and the volume Variation (dV) according to the equation:
| dU = \delta Q - p dV |
and the expression for the second law of thermodynamics with the absolute temperature (T) and the entropy variation (dS) as:
| \delta Q = T dS |
we can conclude that:
| dU = T dS - p dV |
.
ID:(3471, 0)
Enthalpy
Equation 
The enthalpy (H) is defined as the sum of the internal energy (U) and the formation energy. The latter corresponds to the work done in the formation, which is equal to pV with the pressure (p) and the volume (V). Therefore, we have:
ID:(3536, 0)
Differential Enthalpy Relationship
Equation 
The dependency of the differential enthalpy (dH) on the absolute temperature (T) and the entropy variation (dS), in addition to the volume (V) and the pressure Variation (dp), is given by:
If we differentiate the definition of the enthalpy (H), which depends on the internal energy (U), the pressure (p), and the volume (V), given by:
| H = U + p V |
we obtain:
dH = dU + Vdp + pdV
using the differential enthalpy (dH), the internal energy differential (dU), the pressure Variation (dp), and the volume Variation (dV).
By differentiating the internal energy (U) with respect to the absolute temperature (T) and the entropy (S),
| U = T S - p V |
we get:
| dU = T dS - p dV |
with the internal energy differential (dU) and the entropy variation (dS).
Therefore, it finally results in:
| dH = T dS + V dp |
ID:(3473, 0)
Gibbs free energy as differential
Equation 
The dependency of the variation of Gibbs Free Energy (dG) on the entropy (S) and the temperature variation (dT), in addition to the volume (V) and the pressure Variation (dp), is given by:
The gibbs free energy (G) as a function of the enthalpy (H), the entropy (S), and the absolute temperature (T) is expressed as:
| G = H - T S |
The value of the differential of the Gibbs free energy (dG) is determined using the differential enthalpy (dH), the temperature variation (dT), and the entropy variation (dS) through the equation:
dG=dH-SdT-TdS
Since the differential enthalpy (dH) is related to the volume (V) and the pressure Variation (dp) as follows:
| dH = T dS + V dp |
It follows that the differential enthalpy (dH), the entropy variation (dS), and the pressure Variation (dp) are interconnected in the following manner:
| dG =- S dT + V dp |
ID:(3541, 0)
Clausius Clapeyron's law
Equation 
The Clausius-Clapeyron law establishes a relationship between the pressure Variation (dp) and the temperature variation (dT) with the latent Heat (L), the absolute temperature (T) and the volume variation in phase change (\Delta V) as follows:
If the differential of the Gibbs free energy (dG) is constant, it means that for the pressure Variation (dp) and the temperature variation (dT), the values of the entropy (S) and the volume (V) in phase 1
dG = -S_1dT+V_1dp
and the entropy (S) and the volume (V) in phase 2
dG = -S_2dT+V_2dp
yield
\displaystyle\frac{dp}{dT}=\displaystyle\frac{S_2-S_1}{V_2-V_1}
The change in the entropy (S) between both phases corresponds to the latent Heat (L) divided by the absolute temperature (T):
S_2 - S_1 =\displaystyle\frac{ L }{ T }
So, with the definition of the volume variation in phase change (\Delta V)
\Delta V \equiv V_2 - V_1
we obtain
| \displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T } |
ID:(12824, 0)
Molar latent heat conversion
Equation 
In many cases the latent molar heat is not available but the latent heat that is expressed, for example, in Joules per kilogram (J/Kg). Since the vapor pressure equation works with the latent molar heat we must convert the latent heat into latent molar heat. Since the latter is per mole, it is enough to divide the latent heat
In the case of water, the latent heat of evaporation is of the order of
ID:(9273, 0)
Molar Clausius Clapeyron law
Equation 
The Clausius-Clapeyron equation establishes a relationship between the pressure Variation (dp) and the temperature variation (dT) with the absolute temperature (T), the molar Latent Heat (l_m) and the variation of molar volume during phase change (\Delta v_m) as follows:
With the Clausius-Clapeyron law, which depends on the pressure Variation (dp), the temperature variation (dT), the latent Heat (L), the volume variation in phase change (\Delta V), and the absolute temperature (T), expressed as:
| \displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T } |
and the definition of the molar Latent Heat (l_m), where the latent Heat (L) is related to the molar Mass (M_m) as follows:
| l_m \equiv\displaystyle\frac{ L }{ M_m } |
and the variation of molar volume during phase change (\Delta v_m), where the volume variation in phase change (\Delta V) is related to the molar Mass (M_m) as follows:
| \Delta v_m =\displaystyle\frac{ \Delta V }{ M_m } |
we obtain:
| \displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ l_m }{ \Delta v_m T } |
ID:(12822, 0)
Amount of water vapor
Concept 
When the volume variation in phase change (\Delta V) changes phase from a liquid to a gas, it can be expressed as:
\Delta V = V_{\text{gas}} - V_{\text{liquid}}
Since the volume of the gas is significantly greater than that of the liquid,
V_{\text{gas}} \gg V_{\text{liquid}}
we can approximate:
\Delta V \approx V_{\text{gas}}
Given that water vapor behaves similarly to an ideal gas, we can state that with the values of the universal gas constant (R), the number of moles (n), the absolute temperature (T), and the water vapor pressure unsaturated (p_v):
Therefore, the volume variation in phase change (\Delta V) is:
\Delta V = \displaystyle\frac{nRT}{p_v}
ID:(3185, 0)
Pressure saturated water vapor
Equation 
The pressure saturated water vapor (p_s) can be calculated using the reference pressure (p_{ref}), the universal gas constant (R), the absolute temperature (T) and the molar Latent Heat (l_m) according to the following formula:
Using the Clausius-Clapeyron equation for the gradient of the pressure (p) with respect to the absolute temperature (T), which depends on the latent Heat (L) and the volume variation in phase change (\Delta V):
| \displaystyle\frac{ dp }{ dT }=\displaystyle\frac{ L }{ \Delta V T } |
In the case of the phase change from liquid to gas, we can assume that the change in volume is approximately equal to the volume of the vapor. Therefore, we can employ the ideal gas equation with the number of moles (n), the volume (V), the universal gas constant (R), and the water vapor pressure unsaturated (p_v):
| $$ |
Since the Clausius-Clapeyron equation can be written as:
\displaystyle\frac{dp}{dT}=\displaystyle\frac{L}{n}\displaystyle\frac{p}{R T^2}
Where the molar Latent Heat (l_m) (l_m = L/n) corresponds to the change in enthalpy during the phase change h (the energy required to form water), we finally have:
\displaystyle\frac{dp}{dT}=l_m\displaystyle\frac{p}{RT^2}
If we integrate this equation between the pressure saturated water vapor (p_s) and the pressure at point
p_s=p_0e^{l_m/RT_0}e^{-l_m/RT}
If we evaluate this expression with the data at the critical point:
p_{ref}=p_0e^{l_m/RT_0}
We finally have:
| p_s = p_{ref} e^{- l_m / R T } |
ID:(3182, 0)
Relative humidity, concentration
Equation 
The relationship between the concentration of water vapor molecules (c_v) and saturated water vapor concentration (c_s) is referred to as the relative humidity (RH). In other words, when a relative humidity of 100% is reached, the existing concentration will be equal to the saturated concentration.
ID:(3175, 0)
Pressure as a function of molar concentration
Equation 
The pressure (p) can be calculated from the molar concentration (c_m) using the absolute temperature (T), and the universal gas constant (R) as follows:
When the pressure (p) behaves as an ideal gas, satisfying the volume (V), the number of moles (n), the absolute temperature (T), and the universal gas constant (R), the ideal gas equation:
| p V = n R T |
and the definition of the molar concentration (c_m):
| c_m \equiv\displaystyle\frac{ n }{ V } |
lead to the following relationship:
| p = c_m R T |
ID:(4479, 0)
Presión Vapor de Agua
Equation 
The relative humidity (RH) can be expressed in terms of the water vapor pressure unsaturated (p_v) and the pressure saturated water vapor (p_s) as follows:
The relationship between the relative humidity (RH) with the concentration of water vapor molecules (c_v) and saturated water vapor concentration (c_s) is expressed as:
| RH =\displaystyle\frac{ c_v }{ c_s } |
and by relating the pressure (p) with the molar concentration (c_m), the absolute temperature (T), and the universal gas constant (R), we obtain:
| p = c_m R T |
This applies to the vapor pressure of water, where:
p_v = c_v R T
and the saturated vapor pressure of water:
p_s = c_s R T
resulting in the following equation:
| RH =\displaystyle\frac{ p_v }{ p_s } |
ID:(4478, 0)
