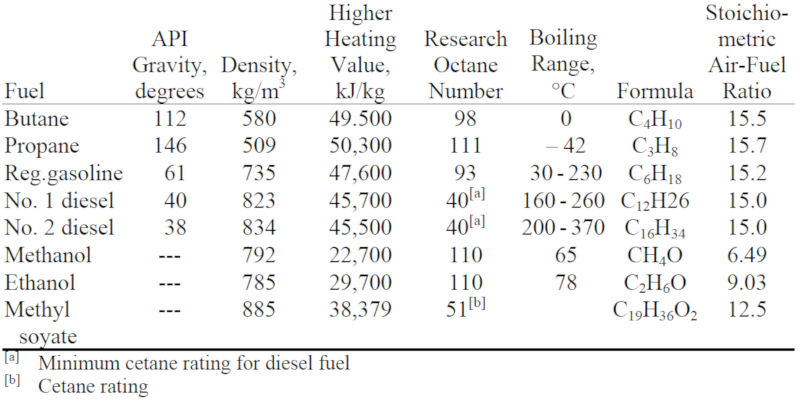
Technical Solution
Concept 
The Otto engine operates in two cycles: the actual Otto cycle, which consists of the following phases:
• Phase 1 to 2: Adiabatic compression
• Phase 2 to 3: Heating
• Phase 3 to 4: Adiabatic expansion
• Phase 4 to 1: Cooling
In addition, it has a cycle for emptying the burnt gases and filling with a fresh mixture.
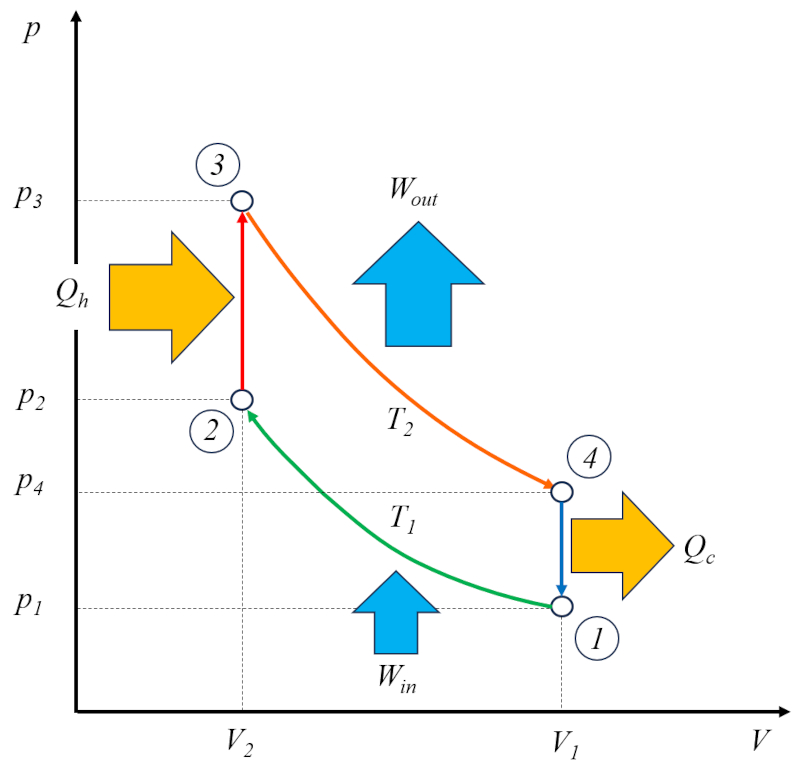
For this reason, it is referred to as a two-stroke engine. The emptying and filling phase can be accomplished using a compensating mass or through a second cylinder that operates out of phase.
The efficiency the efficiency (\eta) of the engine can be estimated using the otto compressibility factor (r) and the adiabatic index (\kappa) with the following equation:
| \eta = 1-\displaystyle\frac{1}{ r ^{ \kappa -1}} |
ID:(11142, 0)
Compressibility factor r
Equation 
The efficiency (\eta) is ultimately a function of the expanded volume (V_1) and the compressed volume (V_2), and in particular, of the otto compressibility factor (r):
Adiabatic expansion is described using the variables the adiabatic index (\kappa), the temperature in state 4 (T_4), the temperature in state 3 (T_3), the expanded volume (V_1), and the compressed volume (V_2) through the relationship
| T_4 V_1 ^{ \kappa - 1} = T_3 V_2 ^{ \kappa - 1} |
While adiabatic compression is represented by the temperature in state 1 (T_1) and the temperature in state 2 (T_2) through the relationship
| T_1 V_1 ^{ \kappa - 1} = T_2 V_2 ^{ \kappa - 1} |
By subtracting the second equation from the first, we obtain
(T_4 - T_1)V_1^{\kappa-1} = (T_3 - T_2)V_2^{\kappa-1}
Which leads us to the relationship
\left(\displaystyle\frac{V_1}{V_2}\right)^{\kappa-1} = \displaystyle\frac{T_3 - T_2}{T_4 - T_1}
And this allows us to define the otto compressibility factor (r) as follows:
| r =\displaystyle\frac{ V_1 }{ V_2 } |
ID:(11162, 0)
Efficiency depending on the compressibility factor
Equation 
The efficiency (\eta) can be calculated from the otto compressibility factor (r) and the adiabatic index (\kappa) in the case of the Otto cycle using:
The efficiency (\eta), in terms of the temperature in state 1 (T_1), the temperature in state 2 (T_2), the temperature in state 3 (T_3), and the temperature in state 4 (T_4), is calculated using the equation:
| \eta =1-\displaystyle\frac{ T_4 - T_1 }{ T_3 - T_2 } |
In the case of adiabatic expansion, it is described using the adiabatic index (\kappa), the expanded volume (V_1), and the compressed volume (V_2) with the relationship:
| T_4 V_1 ^{ \kappa - 1} = T_3 V_2 ^{ \kappa - 1} |
And adiabatic compression is represented by the relationship:
| T_1 V_1 ^{ \kappa - 1} = T_2 V_2 ^{ \kappa - 1} |
If we subtract the second equation from the first, we obtain:
(T_4 - T_1)V_1^{\kappa-1} = (T_3 - T_2)V_2^{\kappa-1}
Which leads to the relationship:
\left(\displaystyle\frac{V_1}{V_2}\right)^{\kappa-1} = \displaystyle\frac{T_3 - T_2}{T_4 - T_1}
This, in turn, leads to the definition of the otto compressibility factor (r) with the equation:
| r =\displaystyle\frac{ V_1 }{ V_2 } |
With all these components, the efficiency of a process using the Otto cycle can be calculated as:
| \eta = 1-\displaystyle\frac{1}{ r ^{ \kappa -1}} |
.
ID:(11163, 0)