Thermodynamic potentials
Definition 
The thermodynamic potentials correspond to different variations of the internal energy $U$, which can include the energy required to form the system equivalent to the work $pV$, as well as the energy that cannot be used to perform work, which is $TS$.
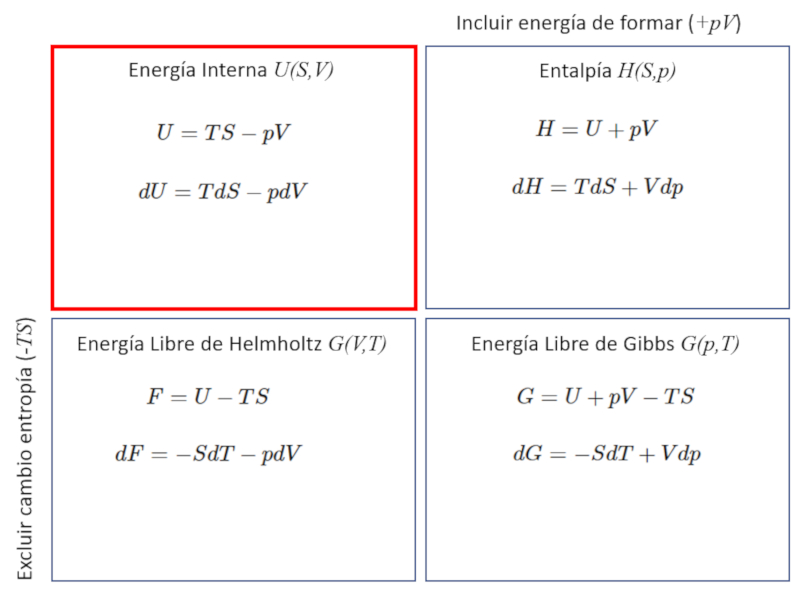
ID:(12348, 0)
