First Law of Thermodynamics
Storyboard 
The first law of thermodynamics states that energy is conserved and consists of two components: one associated with work and the other with heat. Unlike work, this latter component cannot be completely converted into work, which limits the possible energy conversions.
ID:(1398, 0)
Mechanisms
Definition 
The first law of thermodynamics, or the law of energy conservation, states that energy cannot be created or destroyed in an isolated system; it can only be transferred or transformed. This principle asserts that the total energy of an isolated system remains constant. In practical terms, this means the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. Internal energy encompasses the total energy within a system, including kinetic and potential energy at the molecular level. Heat is the energy transferred between systems due to a temperature difference, while work is the energy transferred when a force is applied over a distance, often related to volume changes in gases. The first law is fundamental in analyzing the efficiency of heat engines, the performance of refrigeration and heating systems, and understanding metabolic processes in biological systems. It provides a basis for understanding energy transfers and transformations in various physical processes, ensuring that the energy within an isolated system is always conserved.
ID:(15250, 0)
Energy conservation
Image 
The first law of thermodynamics states that energy is always conserved.
While in mechanics a similar conservation is stated, restricted to non-dissipative systems (for example, excluding friction), thermodynamics generalizes it by considering not only mechanical work but also heat generated or absorbed by the system.
In this sense, the conservation of energy postulated in thermodynamics has no restrictions and is applicable to all systems, as long as all possible energy exchanges and conversions are taken into account.
ID:(37, 0)
First law of thermodynamics
Note 
The first law of thermodynamics was developed through various works [1,2], establishing that energy is conserved. This means that the internal energy differential ($dU$) is always equal to the differential inexact Heat ($\delta Q$) supplied to the system (positive) minus the differential inexact labour ($\delta W$) done by the system (negative).
Therefore, we have:
| $ dU = \delta Q - \delta W $ |
While the exact differential does not depend on how the variation is executed, the inexact differential does. When we refer to a differential without specifying that it is inexact, it is assumed to be exact.
![]() [1] "Über die quantitative und qualitative Bestimmung der Kräfte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842
[1] "Über die quantitative und qualitative Bestimmung der Kräfte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842
![]() [2] "Über die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
[2] "Über die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
ID:(15700, 0)
Pressure and work
Quote 
Let's consider a gas in a cylinder where a piston can move. If the piston is displaced, the volume can be reduced by compressing the gas. To perform this compression, energy is required, which is equal to the force exerted by the gas multiplied by the distance traveled by the piston. This energy can also be represented in terms of pressure since pressure is defined by the force and the area of the piston.
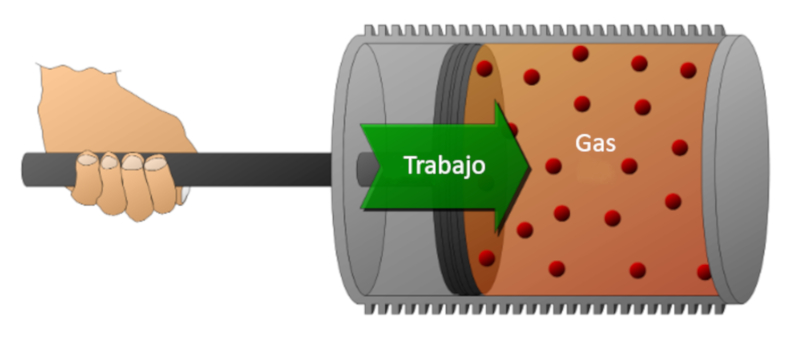
Work can be done on the system (compression) or by the system on the external environment (expansion).
Since the mechanical force ($F$) divided by the section ($S$) is equal to the pressure ($p$):
| $ p \equiv\displaystyle\frac{ F }{ S }$ |
and the volume Variation ($\Delta V$) with the distance traveled ($dx$) is equal to:
| $ \Delta V = S \Delta s $ |
The equation for the differential inexact labour ($\delta W$) can be expressed as:
| $ \delta W = F dx $ |
So it can be written as:
| $ \delta W = p dV $ |
ID:(11126, 0)
First law of Thermodynamics and Pressure
Exercise 
Since the internal energy differential ($dU$) relates to the differential inexact Heat ($\delta Q$) and the differential inexact labour ($\delta W$) as shown below:
| $ dU = \delta Q - \delta W $ |
And it is known that the differential inexact labour ($\delta W$) is related to the pressure ($p$) and the volume Variation ($\Delta V$) as follows:
| $ \delta W = p dV $ |
Therefore, we can conclude that:
| $ dU = \delta Q - p dV $ |
ID:(15701, 0)
First Law of Thermodynamics
Description 
The first law of thermodynamics states that energy is conserved and consists of two components: one associated with work and the other with heat. Unlike work, this latter component cannot be completely converted into work, which limits the possible energy conversions.
Variables
Calculations
Calculations
Equations
(ID 3202)
Since the mechanical force ($F$) divided by the section ($S$) is equal to the pressure ($p$):
| $ p \equiv\displaystyle\frac{ F }{ S }$ |
and the volume Variation ($\Delta V$) with the distance traveled ($dx$) is equal to:
| $ \Delta V = S \Delta s $ |
The equation for the differential inexact labour ($\delta W$) can be expressed as:
| $ \delta W = F dx $ |
So it can be written as:
| $ \delta W = p dV $ |
(ID 3468)
(ID 3469)
Since the internal energy differential ($dU$) relates to the differential inexact Heat ($\delta Q$) and the differential inexact labour ($\delta W$) as shown below:
| $ dU = \delta Q - \delta W $ |
And it is known that the differential inexact labour ($\delta W$) is related to the pressure ($p$) and the volume Variation ($\Delta V$) as follows:
| $ \delta W = p dV $ |
Therefore, we can conclude that:
| $ dU = \delta Q - p dV $ |
(ID 3470)
(ID 15700)
(ID 15701)
Examples
The first law of thermodynamics, or the law of energy conservation, states that energy cannot be created or destroyed in an isolated system; it can only be transferred or transformed. This principle asserts that the total energy of an isolated system remains constant. In practical terms, this means the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. Internal energy encompasses the total energy within a system, including kinetic and potential energy at the molecular level. Heat is the energy transferred between systems due to a temperature difference, while work is the energy transferred when a force is applied over a distance, often related to volume changes in gases. The first law is fundamental in analyzing the efficiency of heat engines, the performance of refrigeration and heating systems, and understanding metabolic processes in biological systems. It provides a basis for understanding energy transfers and transformations in various physical processes, ensuring that the energy within an isolated system is always conserved.
(ID 15250)
The first law of thermodynamics states that energy is always conserved.
While in mechanics a similar conservation is stated, restricted to non-dissipative systems (for example, excluding friction), thermodynamics generalizes it by considering not only mechanical work but also heat generated or absorbed by the system.
In this sense, the conservation of energy postulated in thermodynamics has no restrictions and is applicable to all systems, as long as all possible energy exchanges and conversions are taken into account.
(ID 37)
The first law of thermodynamics was developed through various works [1,2], establishing that energy is conserved. This means that the internal energy differential ($dU$) is always equal to the differential inexact Heat ($\delta Q$) supplied to the system (positive) minus the differential inexact labour ($\delta W$) done by the system (negative).
Therefore, we have:
| $ dU = \delta Q - \delta W $ |
While the exact differential does not depend on how the variation is executed, the inexact differential does. When we refer to a differential without specifying that it is inexact, it is assumed to be exact.
![]() [1] " ber die quantitative und qualitative Bestimmung der Kr fte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842
[1] " ber die quantitative und qualitative Bestimmung der Kr fte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842
![]() [2] " ber die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
[2] " ber die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
(ID 15700)
Let's consider a gas in a cylinder where a piston can move. If the piston is displaced, the volume can be reduced by compressing the gas. To perform this compression, energy is required, which is equal to the force exerted by the gas multiplied by the distance traveled by the piston. This energy can also be represented in terms of pressure since pressure is defined by the force and the area of the piston.

Work can be done on the system (compression) or by the system on the external environment (expansion).
Since the mechanical force ($F$) divided by the section ($S$) is equal to the pressure ($p$):
| $ p \equiv\displaystyle\frac{ F }{ S }$ |
and the volume Variation ($\Delta V$) with the distance traveled ($dx$) is equal to:
| $ \Delta V = S \Delta s $ |
The equation for the differential inexact labour ($\delta W$) can be expressed as:
| $ \delta W = F dx $ |
So it can be written as:
| $ \delta W = p dV $ |
(ID 11126)
Since the internal energy differential ($dU$) relates to the differential inexact Heat ($\delta Q$) and the differential inexact labour ($\delta W$) as shown below:
| $ dU = \delta Q - \delta W $ |
And it is known that the differential inexact labour ($\delta W$) is related to the pressure ($p$) and the volume Variation ($\Delta V$) as follows:
| $ \delta W = p dV $ |
Therefore, we can conclude that:
| $ dU = \delta Q - p dV $ |
(ID 15701)
(ID 15309)
The internal energy differential ($dU$) is always equal to the amount of the differential inexact Heat ($\delta Q$) supplied to the system (positive) minus the amount of the differential inexact labour ($\delta W$) performed by the system (negative):
| $ dU = \delta Q - \delta W $ |
(ID 9632)
The work variance ($\Delta W$) is defined as the product of the force with constant mass ($F$) and the distance traveled in a time ($\Delta s$):
| $ \Delta W = F \Delta s $ |
(ID 3202)
The water column pressure ($p$) is calculated from the column force ($F$) and the column Section ($S$) as follows:
| $ p \equiv\displaystyle\frac{ F }{ S }$ |
(ID 4342)
If we have a tube with a the section Tube ($S$) moving a distance the tube element ($\Delta s$) along its axis, having displaced the volume element ($\Delta V$), then it is equal to:
| $ \Delta V = S \Delta s $ |
(ID 3469)
The differential inexact labour ($\delta W$) is equal to the pressure ($p$) multiplied by the volume Variation ($\Delta V$):
| $ \delta W = p dV $ |
(ID 3468)
With the first law of thermodynamics, it can be expressed in terms of the internal energy differential ($dU$), the differential inexact Heat ($\delta Q$), the pressure ($p$), and the volume Variation ($\Delta V$) as:
| $ dU = \delta Q - p dV $ |
(ID 3470)
ID:(1398, 0)
