Generalized Force
Storyboard 
The generalized forces (intensive variables) and their corresponding conjugate variables (extensive variables) represent how various microscopic parameters can be calculated from microscopic distributions.
ID:(438, 0)
Examples of intensive and extensive variables
Definition 
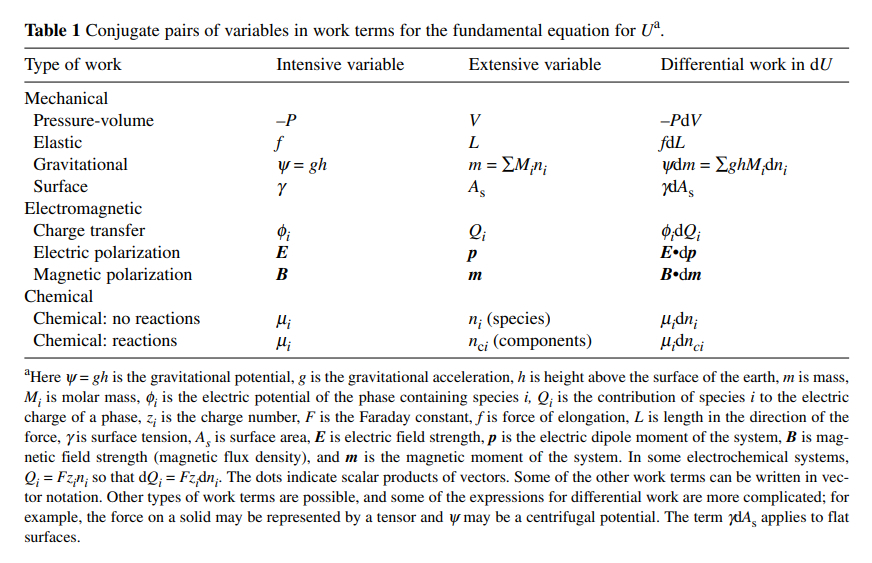
An article that summarizes most of the thermodynamic relationships very well is Use of Legendre Transforms in Chemical Thermodynamics, Robert A. Alberty, Pure Appl. Chem., Vol. 73, No. 8, pp. 13491380, 2001 containing the following table of pairs of extensive and intensive variables:
ID:(11545, 0)
Generalized Force
Storyboard 
The generalized forces (intensive variables) and their corresponding conjugate variables (extensive variables) represent how various microscopic parameters can be calculated from microscopic distributions.
Variables
Calculations
Calculations
Equations
Examples
If we expand the energy around a variable $x_i$:
$dE = -\displaystyle\frac{\partial E}{\partial x_i}dx_i$
we recognize that the derivative of energy with respect to this variable acts as a force that tends to resist changes in the variable. For this reason, the derivative of force with respect to the variable $x_i$, with
is called the generalized force. The generalized force is an intensive variable (it does not depend on the system's size), while the associated variable is an extensive variable (it depends on the system's size).
An example of an extensive variable is volume. When we consider a larger system, its volume increases. However, pressure is intensive, meaning it does not increase when considering a larger system.
As the generalized force e with
can be rewritten as
using the following equation:
$X_i =\displaystyle\frac{\partial E}{\partial x_i}=\displaystyle\frac{\partial E}{\partial S}\displaystyle\frac{\partial S}{\partial x_i}=T\displaystyle\frac{\partial}{\partial x_i} (k\ln\Omega)$
which results in
with
An article that summarizes most of the thermodynamic relationships very well is Use of Legendre Transforms in Chemical Thermodynamics, Robert A. Alberty, Pure Appl. Chem., Vol. 73, No. 8, pp. 13491380, 2001 containing the following table of pairs of extensive and intensive variables:
An example of an extensive variable and generalized force is the volume $V$ with pressure $p$. In this case, the relationship for the generalized force is given with
Si empleamos el numero de estados para el caso de un gas ideal tendremos que el numero de estados es con
$\displaystyle\frac{\partial \ln\Omega}{\partial V}=\displaystyle\frac{N}{V}$
por lo que se obtiene con
ID:(438, 0)
