Balance Condition and Temperature
Storyboard 
To model systems using statistical mechanics, we need to investigate how statistical ensembles can be influenced by the parameters that describe the macroscopic system. For particles, temperature is established as a parameter that reflects whether systems are in equilibrium, maintaining their energies at a constant level.
ID:(436, 0)
A System in contact with a reservoir
Definition 
We can study what happens when we put two systems of particles in contact in such a way that they can exchange energy but not particles.
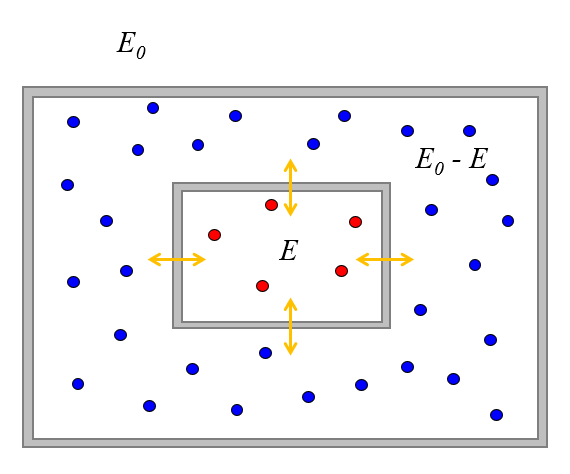
Let's also assume that the system is isolated from the surroundings, meaning it has a total energy of $E_0$.
Suppose initially the first system has an energy of $E$, which is associated with $\Omega(E)$ states.
Since the total energy is $E_0$, the second system can only have the energy $E_0-E" and a number of associated states $\Omega(E_0-E)$.
Once we bring them into contact, they can exchange energy until they reach some equilibrium. In this regard, the value of $E$ will vary, and the probability of finding the systems such that the first one has a value of $E$ will also vary.
ID:(11541, 0)
Comparing the number of state curves
Image 
When we compare how the number of states varies with energy $E$, we observe that the behavior of the system and the reservoir is opposite:

This happens because as the energy increases, the energy of the reservoir decreases, leading to a reduction in the number of states it can access.
ID:(11542, 0)
Forming a maximum
Note 
When we multiply the number of cases, we obtain a function with a very pronounced peak.

The system is more likely to be found at the energy where the peak of the probability curve occurs.
ID:(11543, 0)
Balance Condition and Temperature
Storyboard 
To model systems using statistical mechanics, we need to investigate how statistical ensembles can be influenced by the parameters that describe the macroscopic system. For particles, temperature is established as a parameter that reflects whether systems are in equilibrium, maintaining their energies at a constant level.
Variables
Calculations
Calculations
Equations
Examples
We can study what happens when we put two systems of particles in contact in such a way that they can exchange energy but not particles.
Let's also assume that the system is isolated from the surroundings, meaning it has a total energy of $E_0$.
Suppose initially the first system has an energy of $E$, which is associated with $\Omega(E)$ states.
Since the total energy is $E_0$, the second system can only have the energy $E_0-E" and a number of associated states $\Omega(E_0-E)$.
Once we bring them into contact, they can exchange energy until they reach some equilibrium. In this regard, the value of $E$ will vary, and the probability of finding the systems such that the first one has a value of $E$ will also vary.
Each system $\Omega$ has a number of possible states that depend on its energy $E$. Therefore, if the system we are studying has an energy $E$, the number of possible states will be $\Omega(E)$.
The system under study is in contact with a reservoir that provides energy $E$, so the total energy is $E_0$ minus the immersed system's energy, $E$. Therefore, the reservoir has $\Omega(E_0 - E)$ possible states. The probability of finding the total system with an energy $E$ in the immersed system is expressed as the product of the number of states with
where $C$ is a normalization constant. The energy $E$ will be the one for which the probability is maximum.
When we compare how the number of states varies with energy $E$, we observe that the behavior of the system and the reservoir is opposite:
This happens because as the energy increases, the energy of the reservoir decreases, leading to a reduction in the number of states it can access.
When we multiply the number of cases, we obtain a function with a very pronounced peak.
The system is more likely to be found at the energy where the peak of the probability curve occurs.
If the probability of two isolated systems, each with a total energy of $E_0$ and one of the systems having an energy of $E$, is given by
We can estimate the probable energy $E$ at which they will be found by seeking the maximum probability. To do this, we need to take the derivative with respect to energy $E$ and set the derivative equal to zero.
$\displaystyle\frac{\partial P}{\partial E} = \displaystyle\frac{\partial\Omega}{\partial E}\Omega' + \Omega\displaystyle\frac{\partial\Omega'}{\partial E} = 0$
If we divide the expression by $\Omega\Omega'$ and replace the energy difference $E_0-E$ with $E'$, we can rewrite the condition to determine the most probable situation as follows:
If there is a probability $P(E)$ of finding
$\displaystyle\frac{1}{\Omega}\displaystyle\frac{\partial\Omega}{\partial E} - \displaystyle\frac{1}{\Omega'}\displaystyle\frac{\partial\Omega'}{\partial E'} = 0$
The negative sign arises from the change of variables, as with
$E' = E_0-E$
the derivative with respect to $E'$ results in
When a system is in contact with an energy reservoir $E_0$, it is likely to be found with an energy $E$ for which the probability with
reaches its maximum. The energy can be determined by taking the derivative of this expression with respect to energy $E$ and setting it equal to zero. This is equivalent to taking the derivative of the logarithm of the probability:
$\ln P(E) = \ln C + \ln\Omega(E) + \ln\Omega(E_0-E)$
Leading to:
$\displaystyle\frac{\partial\ln\Omega}{\partial E} + \displaystyle\frac{\partial\ln\Omega}{\partial E} = 0$
If we make a change of variable:
$E' = E_0 - E$
We obtain the equilibrium condition with
The equilibrium condition of a system in contact with a reservoir is expressed with
This allows us to introduce a function $\beta$ with
This function characterizes the state of the system and becomes relevant when the system is in equilibrium with another system.
When a system is in contact with an energy reservoir $E_0$, it is likely to be found with an energy $E$ for which, with
reaches its maximum. The energy can be determined by taking the derivative of this expression with respect to energy $E$ and setting it equal to zero. This is equivalent to taking the derivative of the logarithm of the probability:
$\ln P(E) = \ln C + \ln\Omega(E) + \ln\Omega(E_0-E)$
Thus, with
If we perform a change of variable
$E' = E_0 - E$
we obtain the equilibrium condition with
If we assume that we find the system at the energy for which the probability is maximum, we can associate this with the equilibrium situation of a system, where the probability is maximum.
On the other hand, we know that two systems are in thermal equilibrium if their temperatures are equal. Therefore, the fact that the functions $\beta$ are equal suggests that $\beta$ is related to temperature.
Since the units of $\beta$ are the reciprocal of energy, we can define it as follows with
By introducing the relationship with
the equilibrium condition with
is simplified to just
ID:(436, 0)
