Thermodynamic potentials
Description 
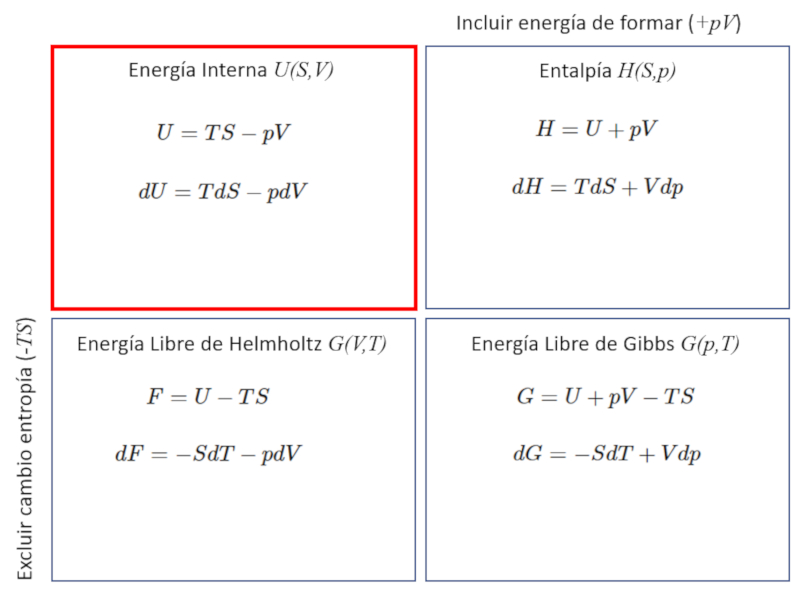
The thermodynamic potentials correspond to different variations of the internal energy $U$, which can include the energy required to form the system equivalent to the work $pV$, as well as the energy that cannot be used to perform work, which is $TS$.
ID:(12348, 0)
Internal energy
Concept 
If the absolute temperature ($T$) and the pressure ($p$) are kept constant, the variation of the internal energy ($dU$), which depends on the entropy variation ($dS$) and the volume Variation ($\Delta V$), is expressed as:
| $ dU = T dS - p dV $ |
Integrating this results in the following expression in terms of the internal energy ($U$), the entropy ($S$), and the volume ($V$):
| $ U = T S - p V $ |
![]() [1] "Über die quantitative und qualitative Bestimmung der Kräfte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842
[1] "Über die quantitative und qualitative Bestimmung der Kräfte" (On the Quantitative and Qualitative Determination of Forces), Julius Robert von Mayer, Annalen der Chemie und Pharmacie, 1842![]() [2] "Über die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
[2] "Über die Erhaltung der Kraft" (On the Conservation of Force), Hermann von Helmholtz, 1847
ID:(214, 0)
Enthalpy
Concept 
The enthalpy ($H$) refers to the energy contained within a system, including any energy required to create it. It is composed of the internal energy ($U$) and the work necessary to form the system, which is represented as $pV$ where the pressure ($p$) and the volume ($V$) are involved.
It is a function of the entropy ($S$) and the pressure ($p$), allowing it to be expressed as $H = H(S,p)$ and it follows the following mathematical relation:
| $ H = U + p V $ |
An article that can be considered as the origin of the concept, although it does not include the definition of the name, is:![]() [1] "Memoir on the Motive Power of Heat, Especially as Regards Steam, and on the Mechanical Equivalent of Heat," written by Benoît Paul Émile Clapeyron (1834).
[1] "Memoir on the Motive Power of Heat, Especially as Regards Steam, and on the Mechanical Equivalent of Heat," written by Benoît Paul Émile Clapeyron (1834).
ID:(215, 0)
Helmholtz free energy
Concept 
The helmholtz free fnergy ($F$) [1] refers to the energy contained within a system, but excludes the energy that cannot be used to perform work. In this sense, it represents the energy available to do work as long as it does not include the energy required to form the system. It is composed, therefore, of the internal energy ($U$), from which the thermal energy $ST$, where the entropy ($S$) and the absolute temperature ($T$) are involved, is subtracted.
This function depends on the absolute temperature ($T$) and the volume ($V$), allowing it to be expressed as $F = F(V,T)$, and it satisfies the following mathematical relation:
| $ F = U - T S $ |
![]() [1] "Über die Thermodynamik chemischer Vorgänge" (On the thermodynamics of chemical processes.), Hermann von Helmholtz, Dritter Beitrag. Offprint from: ibid., 31 May, (1883)
[1] "Über die Thermodynamik chemischer Vorgänge" (On the thermodynamics of chemical processes.), Hermann von Helmholtz, Dritter Beitrag. Offprint from: ibid., 31 May, (1883)
ID:(216, 0)
Gibbs free energy
Concept 
The gibbs free energy ($G$) refers to the energy contained within a system, including the energy required for its formation, but excludes the energy that cannot be used to do work. In this sense, it represents the energy available to do work in a process that includes the energy required for its formation. It is composed, therefore, of the enthalpy ($H$), from which the thermal energy $ST$, where the entropy ($S$) and the absolute temperature ($T$) are involved, is subtracted.
This function depends on the absolute temperature ($T$) and the pressure ($p$), allowing it to be expressed as $G = G(T,p)$ and it satisfies the following mathematical relation:
| $ G = H - T S $ |
![]() [1] "On the Equilibrium of Heterogeneous Substances," J. Willard Gibbs, Transactions of the Connecticut Academy of Arts and Sciences, 3: 108-248 (October 1875 May 1876)
[1] "On the Equilibrium of Heterogeneous Substances," J. Willard Gibbs, Transactions of the Connecticut Academy of Arts and Sciences, 3: 108-248 (October 1875 May 1876)![]() [2] "On the Equilibrium of Heterogeneous Substances," J. Willard Gibbs, Transactions of the Connecticut Academy of Arts and Sciences, 3: 343-524 (May 1877 July 1878)
[2] "On the Equilibrium of Heterogeneous Substances," J. Willard Gibbs, Transactions of the Connecticut Academy of Arts and Sciences, 3: 343-524 (May 1877 July 1878)
ID:(217, 0)
